Step-by-step explanation:
According to principle any change in temperature, pressure or concentration will shift the equilibrium in the direction opposing the change.
For example, in the reaction
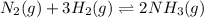 when more ammonia gas is added that is the concentration of products is increasing as ammonia gas is on right hand side.
when more ammonia gas is added that is the concentration of products is increasing as ammonia gas is on right hand side.
Thus, we can conclude that equilibrium will shift on left side that is, on the side of reactants.