Hello!
Three nails have a mass of 3.05 g. How many moles of iron do they contain?
Data:
3 nails of iron = 3.05 g (by nail)
The atomic mass of iron is 55.845 amu, so the molar mass is:
M = 55.845 → M ≈ 56 g / mol
With a simple 3 rule:
56 g → 1 mol
3.05 g → y mol
Therefore:
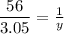
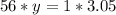
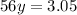
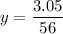
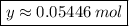
Soon:
3 nails → 3*0.05446 = 0.1638 moles (answer)
_______________________
I Hope this helps, greetings ... Dexteright02! =)