Answer:
The solubility product of cerium(III) hydroxide in the given solution is
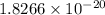 .
.
Step-by-step explanation:
Concentration of cesium hydroxide =
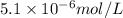
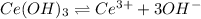
S 3S
1 mol of cerium(III) hydroxide gives 1 mol of cerium ion and 3 moles of hydroxide ions.
Concentration of cerium ion =
![[Ce^(3+)] =S](https://img.qammunity.org/2018/formulas/chemistry/high-school/reks3hg9sy8xnfuaesse81za83h1urj0pe.png)
Concentration of hydroxide ion =
![[OH^-]= 3S](https://img.qammunity.org/2018/formulas/chemistry/high-school/mo4f54etclzihwbalhe7cmrng1ru7ri7v5.png)
The solubility prodcut will be given as:
![K_(sp)=[Ce^(3+)]^1[OH^-]^3](https://img.qammunity.org/2018/formulas/chemistry/high-school/69qw7sdkn7dl1pldrm91uojhom82kxvccn.png)
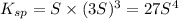
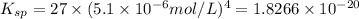
The solubility product of cerium(III) hydroxide in the given solution is
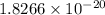 .
.