Hello!
How many molecules are in 30 liters of methane (CH4) at STP ?
We have the following information:
Knowing that by Avogadro's Law for each mole of substance we have 6.02 * 10²³ molecules, it is known that in STP (Standard Temperature and Pressure) one mole of any gas equals 22.4 L, then:
22.4 L ----------------- 6.02*10²³ molecules
30 L -------------------- y molecules
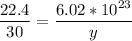
multiply the means by the extremes
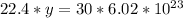
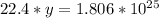
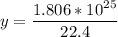
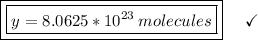
Answer:
8.0625*10²³ molecules of methane
________________________