Answer : (a)
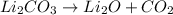 is a decomposition reaction.
is a decomposition reaction.
Explanation :
(a)
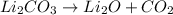 is a decomposition reaction.
is a decomposition reaction.
- Decomposition reaction : It is a type of chemical reaction in which a single compound decomposes into two or more compounds.
(b)
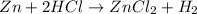 is a displacement reaction.
is a displacement reaction.
- Displacement reaction : It is a type of reaction in which a most reactive element displaces the least reactive element in a compound.
(c)
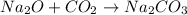 is a combination reaction.
is a combination reaction.
- Combination reaction : It is a type of reaction where two or more compounds combine to form a single compound.
(d)
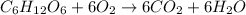 is a combustion reaction.
is a combustion reaction.
- Combustion reaction : It is a type of reaction where a hydrocarbon react with the oxygen to produced carbon dioxide and water.
Therefore, option (a) is a decomposition reaction.