Answer: The correct option is A.
Step-by-step explanation: In a chemical reaction, reactants react to form a number of products.
For the formation of products, the bonds of the individual reactants must be broken and the bonds of the products must be formed.
For example: Formation of water from hydrogen gas and oxygen gas. Reaction follows:
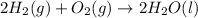
The Bonds of hydrogen and oxygen molecule are broken and new bonds between hydrogen and oxygen atoms are formed to give water molecule.