Answer:
20.268 grams of calcium metal contains the same number of atoms as 12.16 g of magnesium.
Step-by-step explanation:
Atomic mass of magnesium= 24 g/mol
Mass of magnesium given = 12.16 g
Moles of magnesium=
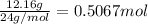
1 mol =
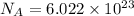 atoms/ molecules, ions
atoms/ molecules, ions
Number of atoms of magnesium in 0.5067 moles of magnesium:
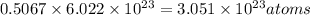
Calcium metal contains the same number of atoms:
Moles of calcium = n
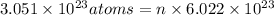
n = 0.5067 mol
Atomic mass of calcium = 40 g/mol
Mass of 0.5067 moles of calcium:
0.5067 mol × 40 g/mol = 20.268 g