Answer:
1.2046*10²⁴ atoms
Step-by-step explanation:
Avogadro's Number or Avogadro's Constant to the number of particles that constitute a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023 * 10²³ particles per mole. Avogadro's number represents a quantity without an associated physical dimension, so it is considered a pure number that allows describing a physical characteristic without dimension or explicit unit of expression. The Avogadro number applies to any substance.
Then a rule of three applies as follows: if 1 mole has 6,023 * 10²³ atoms, 2 moles how many atoms will it contain?
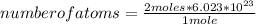
number of atoms=1.2046*10²⁴ atoms
This number is already represented in scientific notation.