Answer: The correct answer is Option 3.
Step-by-step explanation:
Chemical equilibrium is defined as the point where the concentrations of reactants and the products do not change with time.
The equation for the equilibrium between
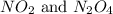 follows:
follows:
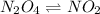
Initially, the concentration of the reactants decrease and the concentration of the products increase as seen from the graph. But after 60 seconds, the concentration of both reactants and products do not change and hence equilibrium is attained.
Hence, the correct answer is Option 3.