Step-by-step explanation:
Aqueous solution of potassium chromate contains mobile ions that freely move from one place to another.
Potassium chromate solution contains charged particles. These charged particles are
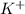 ions and
ions and
 ions.
ions.
Therefore, due to the presence of these charged particles or ions a solution of potassium chromate is able to conduct electricity.