Answer:
Water.
Step-by-step explanation:
Hello,
In this case, you are referring to the dissociation or formation of water at the equilibrium, that is:
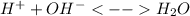
In such a way, the water is the conjugate acid of the hydroxile ion as long as it gains a proton.
Best regards.