Step-by-step explanation:
The given reaction will be as follows.
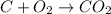
Therefore, according to this equation 1 mole of carbon is reacting with 1 mole of oxygen to give 1 mole of carbon dioxide.
Hence, 0.4 mole of carbon will react with 0.4 mole of oxygen atom which will result in the formation of 0.4 mole of carbon dioxide.
Therefore, carbon is the limiting reagent and oxygen is the excess reagent.
Thus, we can conclude that for the given reaction 0.4 moles of
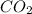 are formed.
are formed.