Answer : The atomic symbol represent an isotope that undergoes radioactive decay to produce Pb-206 can be, Polonium (Po).
Explanation :
Alpha decay : In this process, the alpha particles is emitted when a heavier nuclei decays into lighter nuclei. The alpha particle released has a charge of +2 units.
The general representation of alpha decay,
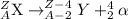
The balanced nuclear reaction for alpha decay of polonium-210,
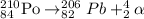
Hence, the atomic symbol represent an isotope that undergoes radioactive decay to produce Pb-206 can be, Polonium (Po).