Answer:
The percentage of barium nitrate in the impure sample is 76.98%.
Step-by-step explanation:
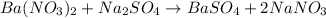
A) When excess of sodium nitrate solution added to barium nitrate solution to precipitate barium sulfate which is white in color. The relevance of an excessive sodium sulfate is to precipitate maximum amount of barium ions dissolved in the solution.
B) Mass of the precipitate i.e barium sulfate = 0.848 g
Moles of barium sulfate =
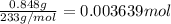
According to reaction, 1 mole of barium sulfate is obtained from 1 mole of barium nitrate.
Then 0.003639 moles of barium sulfate will be obtained from :
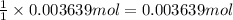 of barium nitrate.
of barium nitrate.
Mass of 0.003639 moles of barium nitrate:
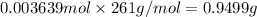
Percentage of barium nitrate in impure sample:
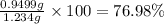
The percentage of barium nitrate in the impure sample is 76.98%.