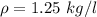Answer:
Step-by-step explanation:
Density
The density of a substance is the mass per unit volume. The density varies with temperature and pressure.
The formula to calculate the density of a substance of mass (m) and volume (V) is:
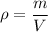
The cube has a mass of m=3.75 g and a volume of V=3 ml, thus the density is:
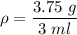

Since 1 kg=1000 mg and 1 lt = 1000 ml, the density has the same value but with different units: