Answer: No, the given elements will not react.
Step-by-step explanation:
Ionic bond is defined as the bond which is formed from the complete transfer of electrons from one atom to another atom. The atom which looses electrons is known as electropositive atom and it attains positive charge. The atom which gains electrons is known as electronegative atom and it attains negative charge. For Example: KBr
Covalent bond is defined as the bond which is formed by the sharing of electrons between the atoms forming a bond. No ions are present in the compound. For Example: HCl
We are given, two elements with their oxidation states:
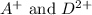
As, both the ions are positive ion and thus, will not react to form any compound because like charges repel each other.
Hence, no, the given elements will not react.