You have a balanced equation that says one mole of
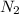
plus three moles of
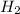
react to produce two moles of
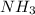
. So if 2 moles of hydrogen are required for 3 moles of ammonia, then you can make the simplest of equations to solve this. That is,
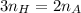
Solving for the number of moles of hydrogen then
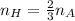
You were given the number of moles of ammonia so plug it in and you are done!