Answer: Four
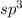 hybridized orbitals will formed when one s-orbital and three p-orbitals combines.
hybridized orbitals will formed when one s-orbital and three p-orbitals combines.
Step-by-step explanation:
- When ever one s-orbital combines with three p-orbitals it gives four
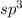 hybridized orbitals.
hybridized orbitals. - In which there is 25% of s character and 75% of p character.
- The shape of the
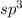 hybridized orbitals is Tetrahedral. As shown in image attached.
hybridized orbitals is Tetrahedral. As shown in image attached.