Answer: The atom having given electronic configuration is sulfur.
Step-by-step explanation:
Electronic configuration is the representation of electrons around the nucleus of an atom. This representation also helps in the determination of atomic number of an element.
We are given:
Electronic configuration of a metal =
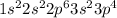
Total number of electrons present in the atom = [2 + 2 + 6 + 2 + 4] = 16
The element having atomic number 16 is sulfur.
Hence, the atom having given electronic configuration is sulfur.