Answer: Option (3) is the correct answer.
Step-by-step explanation:
A molecule is polar when the net dipole moment of molecule is zero. When an electronegative atom is attached to an electropositive atom then the electronegative atom pulls the shared pair of electrons more towards itself.
As a result, the movement of electrons is more towards the electronegative atom and the dipole moment becomes zero. This will also induce partial positive and partial negative charge on electropositive and electronegative atom.
On the other hand,
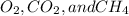 are covalent compounds and does not have a net zero dipole moment.
are covalent compounds and does not have a net zero dipole moment.
Thus, we can conclude that out of the given options
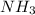 represents a polar molecule.
represents a polar molecule.