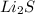Answer:
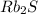 <
<
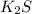 <
<
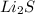
Step-by-step explanation:
Lattice energy is the energy required to dissociate 1 mole of the an ionic compound into its constituent ions.
Thus higher is the lattice energy more energy will be required to melt the compounds.
As
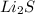 has highest melting point, it will require most energy to melt an hence it will have highest lattice energy. The order of three compounds in order of increasing lattice energy
has highest melting point, it will require most energy to melt an hence it will have highest lattice energy. The order of three compounds in order of increasing lattice energy
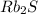 <
<
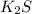 <
<