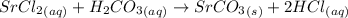We will balance the equation in the following order: metals, amethals, carbon, hydrogen and oxygen (the most common order).
The metal present in the equation is Sr, which is already balanced (there are 1 on each side of the equation).
The amethal present in the equation is Cl. There is 2 Cl in the left side and only one in the right side. So, we will multiply the quantity of the molecule that contains Cl by 2. Doing this, we'll obtain:
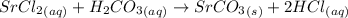
Looking at the equation, we can see that it is now fully balanced. Hence, a balanced equation of the reaction is: