Answer:
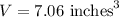
Explanation:
Given hat,
The radius of cone shaped paper cup, r = 1.5 inches
The height of cone shaped paper cup, h = 3 inches
We need to find the volume of the cup. For a cone shaped, the volume is given by :
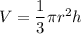
Putting all the values in the above formula.
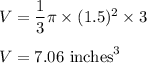
So, the volume of the cup is
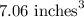 .
.