Answer:
13.122 grams of Al will be left.
Step-by-step explanation:
The balanced possible reaction between Al and S is:
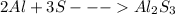
Thus two moles of aluminium will react with three moles of sulfur
the moles of sulfur present =
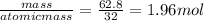
Moles of Al present =
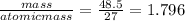
1.96 moles of sulfur will react with =
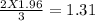 moles of Al
moles of Al
The moles of Al left = The moles of excess reagent left = 1.796-1.31=0.486mol
the mass of Al left = moles X atomic mass =0.486X27=13.122 grams