Answer:
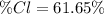
Step-by-step explanation:
Hello,
In this case, chlorine's percent composition is given by the shown below formula:
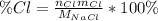
Whereas
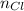 accounts for the number of chlorine atoms into the NaCl,
accounts for the number of chlorine atoms into the NaCl,
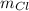 for chlorine's atomic mass and
for chlorine's atomic mass and
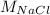 for sodium chloride's molar mass, therefore, the percent composition of chlorine turns out:
for sodium chloride's molar mass, therefore, the percent composition of chlorine turns out:
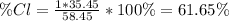
Best regards.