Answer: Average bond dissociation enthalpy of a (Xe–F) bond is 131.5kJ/mol.
Expatiation:
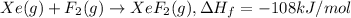 ..(1)
..(1)
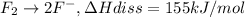 ..(2)
..(2)
Subtracting (1) from (2)
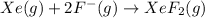
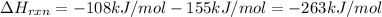
Average bond dissociation enthalpy of a (Xe–F) bond :
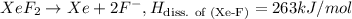
Since there are two (Xe-F) bonds in molecule the average the bond energy of Xe-f bond will be =
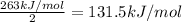
Hence, Average bond dissociation enthalpy of a (Xe–F) bond is 131.5kJ/mol