Answer: Option (d) is the correct answer.
Step-by-step explanation:
Species which contain same number of protons but different number of neutrons are known as isotope.
For example,
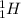 and
and
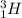 are isotopes.
are isotopes.
More is the mass of particle colliding with the isotope more will be the change in mass of an isotope due to emission of a heavier particle.
As alpha (
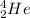 ) particle is heavier then a neutron, positron and gamma particles.
) particle is heavier then a neutron, positron and gamma particles.
For example,
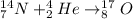
Therefore, we can conclude that alpha particle changes the mass of the isotope the most.