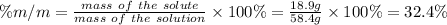Answer:
32.4%
Step-by-step explanation:
Step 1: Given data
Mass of the solute = 18.9 g
Mass of the solvent = 39.5 g
Mass of the solution = Mass of the solute + Mass of the solvent = 58.4 g
Step 2: Calculate the mass percent of the solute in the solution
We use the following expression.