Redox reaction is the chemical reaction in which both oxidation (loss of electrons) and reduction (gain of electrons) takes place or transfer of electrons between two species occurs.
The given chemical equation is :
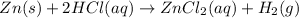
Here, in reactant side, the oxidation state of zinc is zero (0), after reacting with hydrogen chloride, the oxidation sate of zinc becomes two (+2). In this reaction, electrons are transferred from zinc atoms to the hydrogen atoms. Thus, zinc is oxidized by losing electrons.
Hence, oxidation state of zinc increases due to loses electrons i.e. Option (A) is correct.