Answer: Ionic compound is formed by the elements
1) aluminium and oxygen
2) lithium and fluorine
Explanation: Ionic compounds are defined as the compounds in which there is complete transfer of electrons from one element ( a metal) to another element ( a non-metal).
From the given elements, two pairs will result in the formation of ionic compounds.
1) Aluminium and oxygen
In this Aluminium is a metal having +3 oxidation state and oxygen is a non metal having -2 oxidation state. This results in the formation of an ionic compound. The compound formed is
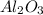
2) Lithium and fluorine
In this, Lithium is a metal having +1 oxidation state and Fluorine is a non-metal having -1 oxidation state which results in the formation of an ionic compound having formula LiF.
One of the rest pair of elements form covalent bond which is oxygen and fluorine. These two elements are non- metals and will result in the formation of covalent bond only. Covalent bond is the sharing of electrons between two elements. The covalent compound formed from these twp elements is
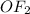
The final pair which is calcium and aluminium will not result in the formation of any compound because both the elements are metals and have positive charge on them, so both the elements will repel each other.