Answer: Bromine is produced at anode.
Step-by-step explanation:
On electrolysis of molten calcium bromide, the following reaction occurs:
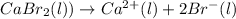
In molten
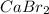 there are
there are
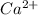 and
and
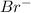 ions.
ions.
At cathode which is a negative terminal , cations will be reduced.At anode which is a positive terminal , anions will be oxidized.
At cathode:
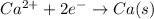
So, calcium is deposited at cathode.
At anode:
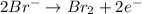
At anode
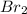 is produced.
is produced.
Thus bromine is produced at anode.