Radioactive elements obey 1st order kinetics,
For 1st order reaction, relation between rate constant (k) and half life [t(1/2)] is,
k =
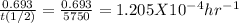
Also, for 1st order reaction, we have
t =
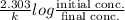
Given that:
the bones from a mastodon had lost 78.5% of their C14,
∴ initial conc. of C14 = 100%, conc. of C14 left after time 't' = 21.5%
∴t =
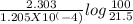 = 1.2758 X 10^4 hours
= 1.2758 X 10^4 hours