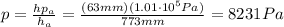We can find the gas pressure by using a simple proportion.
In fact, if the pressure of the gas was equal to the atmospheric pressure:
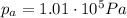
we know that the height in the mercury manometer would have been
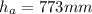
Therefore, if the heigth in the mercury manometer is
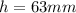
Then the pressure of the gas is given by the following proportion
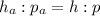
From which we find