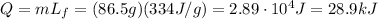The ice is already at melting point, therefore we don't need additional heat to increase its temperatute. Instead, we have to give it the necessary heat to melt it, and the amount of this heat is given by
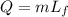
where
m is the mass of the ice
Lf is the latent heat of fusion of ice
For ice,
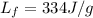
, while the mass of this sample of ice is
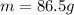
, therefore the amount of heat needed to melt it is