Wavelength and frequency are inversely proportional, therefore the minimum wavelength occurs when the emitted radiation has maximum frequency.
The energy of the emitted photons is
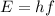
(1)
where h is the Planck constant and f is the frequency. The maximum energy that can be given to the emitted photons corresponds to the kinetic energy of the electrons (in fact, if they are completely stopped, they give all their kinetic energy to the photons). Converting the energy in Joules:
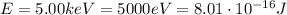
from eq.(1) we find the corresponding frequency of the photons:
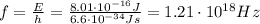
This is the maximum frequency the emitted photons can have, and by using the basic relationship between frequency, wavelength and speed of light c, we can find the corresponding wavelength:
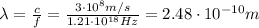
And this is the minimum wavelength of the radiation emitted by this electron beam.