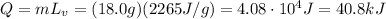The water is already at its boiling point
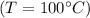
, therefore we don't need additional heat to increase its temperature. Instead, we have to give the heat necessary to make it evaporate. The energy needed is equal to
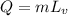
where
m is the mass of the water
Lv is the latent heat of evaporation
The latent heat of evaporation of water is
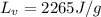
, and the mass of this sample of water is
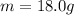
, therefore the heat needed to vaporize the water is