Step-by-step explanation:
Heat of vaporization is defined as the amount of heat required to change one mole of a liquid into vapor state without any change in the temperature.
It is known that for 1 mole of water, latent heat of vaporization is 40.6 kJ/mol.
Therefore, heat of vaporization for 1.30 moles will be calculated as follows.
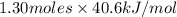
= 52.78 kJ
Thus, we can conclude that the 52.78 kJ of heat are needed to completely vaporize 1.30 moles of
 .
.