Answer : The volume of
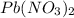 needed are, 4.6875 ml
needed are, 4.6875 ml
Explanation :
First we have to calculate the moles of
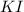 .
.
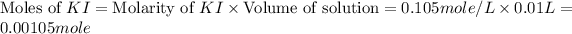
Now we have to calculate the moles of
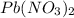
The given balanced chemical reaction is,
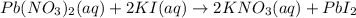
From the balanced chemical reaction, we conclude that
As, 2 moles of KI react with 1 mole of
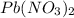
So, 0.00105 moles of KI react with
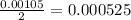 mole of
mole of
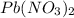
Now we have to calculate the volume of
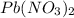
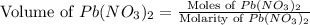
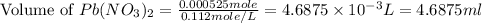
conversion used : (1 L = 1000 ml)
Therefore, the volume of
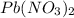 needed are, 4.6875 ml
needed are, 4.6875 ml