Answer:
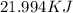
Step-by-step explanation:
The acetone has the following molecular formula :
C3H6O
The molar mass of the acetone is
μ(C3H6O) =
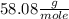
You can find this value in any table.
The molar mass means the mass that has 1 mole of C3H6O
Now, the heat follows this equation :
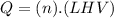
Where Q is the heat, n is the number of moles of the substance and LHV is the latent heat of vaporization of the substance.
Let's calculate n
If 1 mole of C3H6O has a mass of 58.08 g ⇒
x moles of C3H6O will have a mass of 43.9 g ⇒
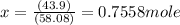
In 43.9 g of C3H6O there is 0.7558 moles of C3H6O
The LHV for C3H6O is
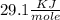
You can find this value in any table
To find the amount of heat :
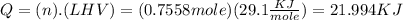
We find out that 21.994 KJ are required to vaporize 43.9 g of acetone (C3H6O).