The energy levels of the hydrogen atom are given by
![E_n = -13.6 (1)/(n^2) [eV]](https://img.qammunity.org/2019/formulas/physics/college/l05fq35v48cg8azrg9sqch628f4tzrlcrx.png)
(1)
where n is the level number. Therefore, the ground state has energy of
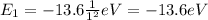
If the atom absorbs a photon of energy
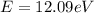
, the final energy of the hydrogen atom is
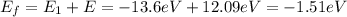
And we can use eq.(1) to find the corresponding level number:
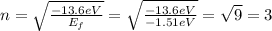
So, the electron made a transition to the n=3 level.