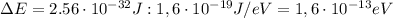We can solve the exercise by using Heisenberg's principle. In its energy-time version, Heisenberg principle states that the product between the uncertainty on the energy and on the time is larger than:
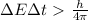
(1)
where
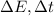
are the uncertainties on the energy and on the time, and h is the Planck constant.
The lifetime of the particle is 2.05 ms, so we can assume the maximum uncertainty on the time corresponds to the lifetime itself:
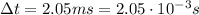
And so the minimum uncertainty on the energy can be found by using (1):
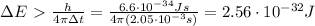
Keeping in mind that
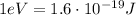
, we can convert the energy uncertainty into electronvolts: