The energy levels of the hydrogen atom are given by
![E_n=-13.6 (1)/(n^2) [eV]](https://img.qammunity.org/2019/formulas/physics/college/3ixtzdpnvsqicst7fvis93cccb5nvy64v5.png)
where n is the level number.
In order to make transition from n=2 state to n=5 state, the electron should acquire an energy equal to the difference between the two energy levels:
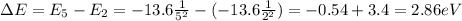
Keeping in mind that
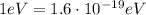
, we can convert this energy in Joules
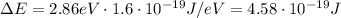
This is the energy the photons of the laser should have in order to excite electrons from n=2 state to n=5 state. Their frequency can be found by using
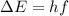
where h is the Planck constant and f is the photon frequency. Re-arranging it, we find
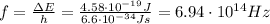
and finally we can use the relationship between frequency, wavelength and speed of light which holds for photons, in order to find their wavelength:
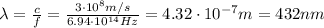
and this is the laser wavelenghth.