Answer:
Concentration of solution in percent by mass is 13.3% CaO
Step-by-step explanation:
% by mass =
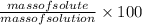
Here solute is CaO and solvent is water.
So, mass of solution = (mass of solute)+(mass of solvent)
= (mass of CaO)+(mass of water)
= 32.5 g + 212 g
= 244.5 g
So, Concentration of solution by percent mass =
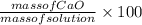 =
=
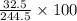 = 13.3 %
= 13.3 %