Answer:
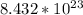 molecules
molecules
Step-by-step explanation:
As per Avogadro's constant for atoms, one mole of an element/compound consists of
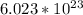 atoms/molecules.
atoms/molecules.
Thus, the number of molecules in “n” moles of an element and compound is equal to the product of number of moles of the element and compound and Avogadro's constant.
Thus, 1.4 moles of
 will have
will have
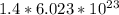 molecules
molecules
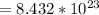 molecules
molecules