Following chemical reaction is involved for the reaction between K3PO4 and NiCl2
2K3PO4 + 3NiCl2 → 6KCl + Ni3(PO4)2
Volume of solutions is converted to litres for present calculations (1litre = 1000ml)
number of moles of NiCl2 = 0.130 × 0.0116
=0.001508 mol
Now,
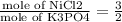
∴ mole of K3PO4 =
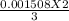
=0.001005 mol
given that, K3PO4 is 0.205 M
i.e. 0.205 mol of K3PO4 is present in 1 litre of solution
then 0.001005 mol is present in X litre of solution
∴X =
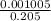
=0.004902 litre =4.902ml