Answer:
4.014 g of water
Step-by-step explanation:
An internet search for your question tells me that the chemical equation is:
- BaH₂(s) + 2H₂O(l) → Ba(OH)₂(aq) + 2H₂(g)
So first we use PV=nRT to calculate the moles of hydrogen gas produced:
- P = 755 mmHg ⇒ 755/760 = 0.993 atm
- T = 25 °C ⇒ 25 + 273.15 = 298.16 K
0.993 atm * 5.50 L = n * 0.082 atm·L·mol⁻¹·K⁻¹ * 298.16 K
Now we convert mol H₂ to mol H₂O and finally to grams of water:
0.223 mol H₂ *
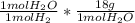 = 4.014 g H₂O
= 4.014 g H₂O