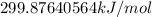The chemical reaction is given as:
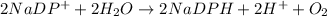
Here, oxygen is oxidised and
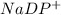 is reduced. Thus, redox reaction occurs.
is reduced. Thus, redox reaction occurs.
For cell reaction,
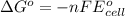 (2)
(2)
where,
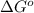 = standard state free energy
= standard state free energy
n= number of electrons
F= Faraday constant (
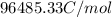 )
)
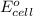 = cell potential
= cell potential
Substitute the value of number of electrons i.e. 2, Faraday constant and cell potential in the formula to determine the value of
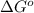 .
.
Now, calculate the value of cell potential
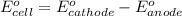 (1)
(1)
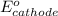 =
=
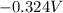 (standard reduction potential of
(standard reduction potential of
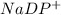 )
)
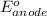 =
=
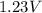 (standard reduction potential of
(standard reduction potential of
 )
)
Put the above values in formula (1), we get:
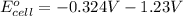
=
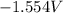
Now, substitute above value in formula (2)
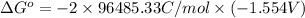
=
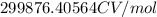
Since, one coulomb volt is equal to one joule.
Thus, value of
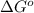 is equal to
is equal to
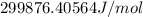 or
or