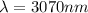The relationship between wavelength, frequency and speed of an electromagnetic wave is given by
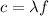
where
c is the speed of light
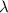
is the wavelength
f is the frequency
The infrared radiation of our problem has frequency
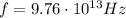
, therefore if we re-arrange the previous equation we can calculate its wavelength:
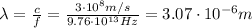
and converted into nanometers,