molar mass is the sum of the products of molar masses of individual elements by the number of atoms of the element in the compound.
molar mass of H - 1.01 g
number of H atoms - 21 H atoms
therefore mass of H in 1 mol of compound - 1.01 g x 21 = 21.21 g
the molar mass of the compound - 303.39 g/mol
H composition percentage =
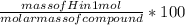
composition percentage = 21.21 g/mol /303.39 g/mol x 100%
H composition percentage = 6.991 %
therefore answer is 6.99 %