At
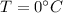
, the volume of the gas is V=22 L.
At temperature
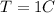
, the volume of the gas is (22+225) L.
This means that if we increase the temperature by
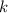
degrees:
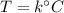
the volume of the gas is
![V=22 + 225 k [L]](https://img.qammunity.org/2019/formulas/mathematics/college/vqi6mr6d6a4bloejrscqdhtbyit54fpc41.png)
(1)
Since T=k, we can rewrite eq.(1) as
![V= 22 + 225 T [L]](https://img.qammunity.org/2019/formulas/mathematics/college/g2967f3pnljykf2jkkd2v15ybro5ha57eu.png)
which gives the expression for the volume of the gas in liters, as a function of the temperature T in Celsius.